Psychedelic Science in 2026: From Fringe Research to Frontline Neuroscience and Psychiatry

Over the last 25 years, psychedelic science has gradually re-entered mainstream neuroscience and psychiatry after decades of regulatory pause. Supported by FDA-regulated clinical trials, advanced neuroimaging, and global academic research, psychedelic-assisted therapies are being rigorously studied for depression, anxiety, PTSD, addiction, and brain health conditions. This article summarizes the evidence, institutions, and clinical progress shaping the field today.
A Brief History of Psychedelic Research
After more than three decades of regulatory pause, psychedelic science has quietly returned to the center of serious neuroscience and psychiatry. In the 1950s and 60s, psychedelics were widely studied across mainstream academic medicine; early research explored their therapeutic potential in patients with depression, alcoholism, existential distress, terminal cancer and other conditions.
This work was largely halted in 1970 with the Controlled Substances Act, when these medicines became entangled with the counterculture movement, political backlash, and broader drug-policy restrictions. Today, after a long hiatus psychedelic science has reemerged, more rigorous, thoughtful, and clinically grounded than ever, driven by modern clinical trials, advanced brain-imaging research, and our rapidly evolving understanding around neuroplasticity.
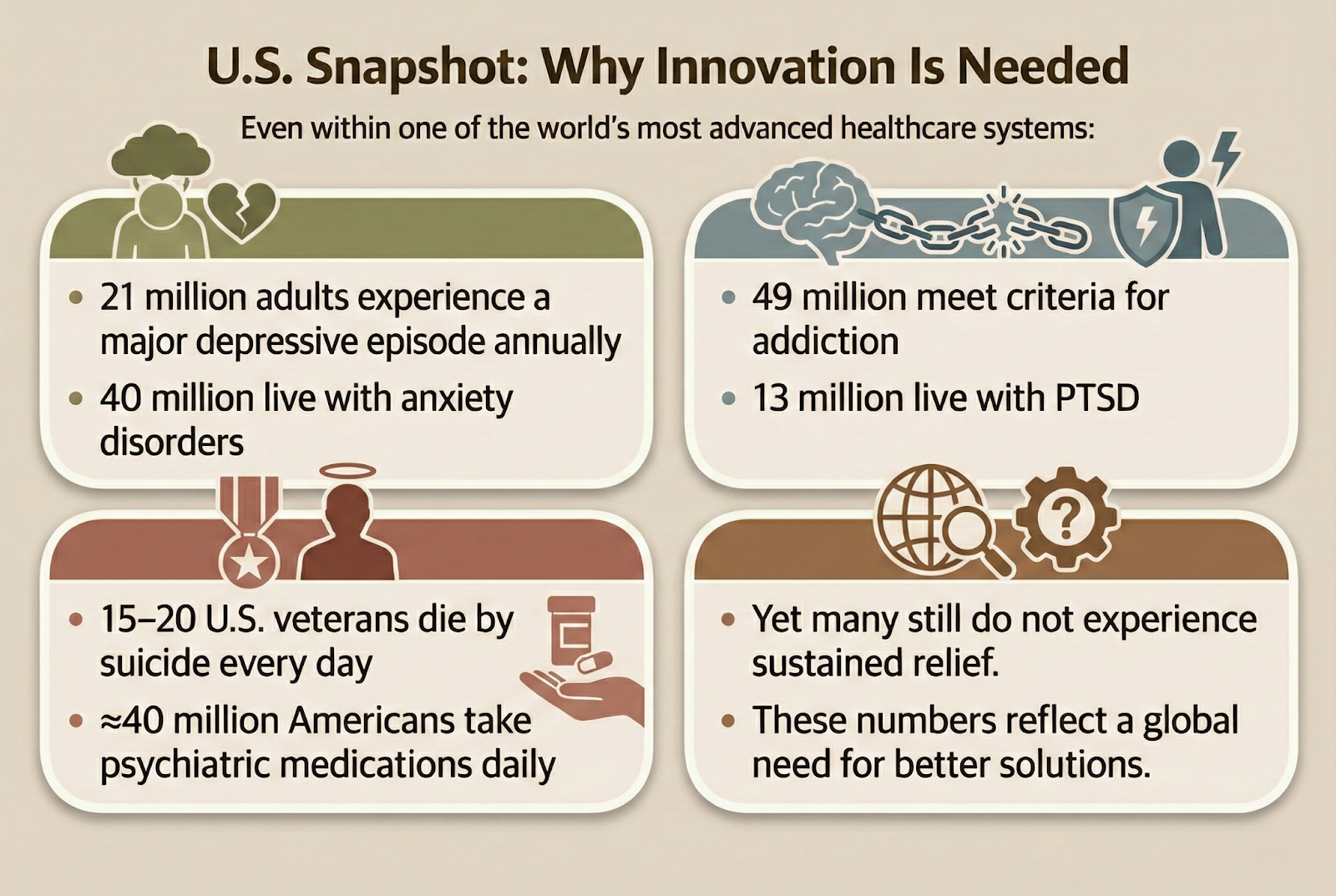
A Global Mental and Brain Health Crisis
Mental health disorders remain among the leading causes of disability worldwide. Depression, anxiety, trauma-related disorders, and addiction continue to impose enormous human and economic costs. Even where treatment is available, many patients do not achieve lasting relief.
At the same time, neurological and brain health conditions demand new approaches. These include mild cognitive impairment (MCI),early dementia, post-stroke cognitive and functional decline, traumatic brain injury (TBI) recovery and chronic pain syndromes
These conditions deeply affect quality of life, family systems, productivity, and human potential. Existing treatments help many but not nearly enough. This urgent reality is why leading institutions across the globe are re-examining psychedelic-assisted therapies as a means to provide enhanced neuroplasticity and potential symptom relief.
From Fringe to Frontline Science
Since the early 1990s, a new generation of researchers reopened human psychedelic studies with heightened safety oversight and scientific rigor. Early clinical findings, particularly with psilocybin and MDMA, demonstrated meaningful and sometimes rapid improvements in depression, anxiety, trauma-related distress, addiction, and end-of-life suffering.
The FDA has recognized the promise of these therapies by granting Breakthrough Therapy designation to:
- MDMA-assisted therapy for PTSD
- Psilocybin-assisted therapy for treatment-resistant depression and major depressive disorder
- LSD for generalized anxiety disorder
This signals federal acknowledgment that these treatments may provide substantial benefit when delivered responsibly.
A Global Research Effort Led by the World’s Top Institutions
One of the clearest indicators that psychedelic medicine is no longer fringe is its global scientific footprint. Today there are over 50 major research institutions world-wide in North America, Europe and elsewhere advancing psychedelic science. These include:
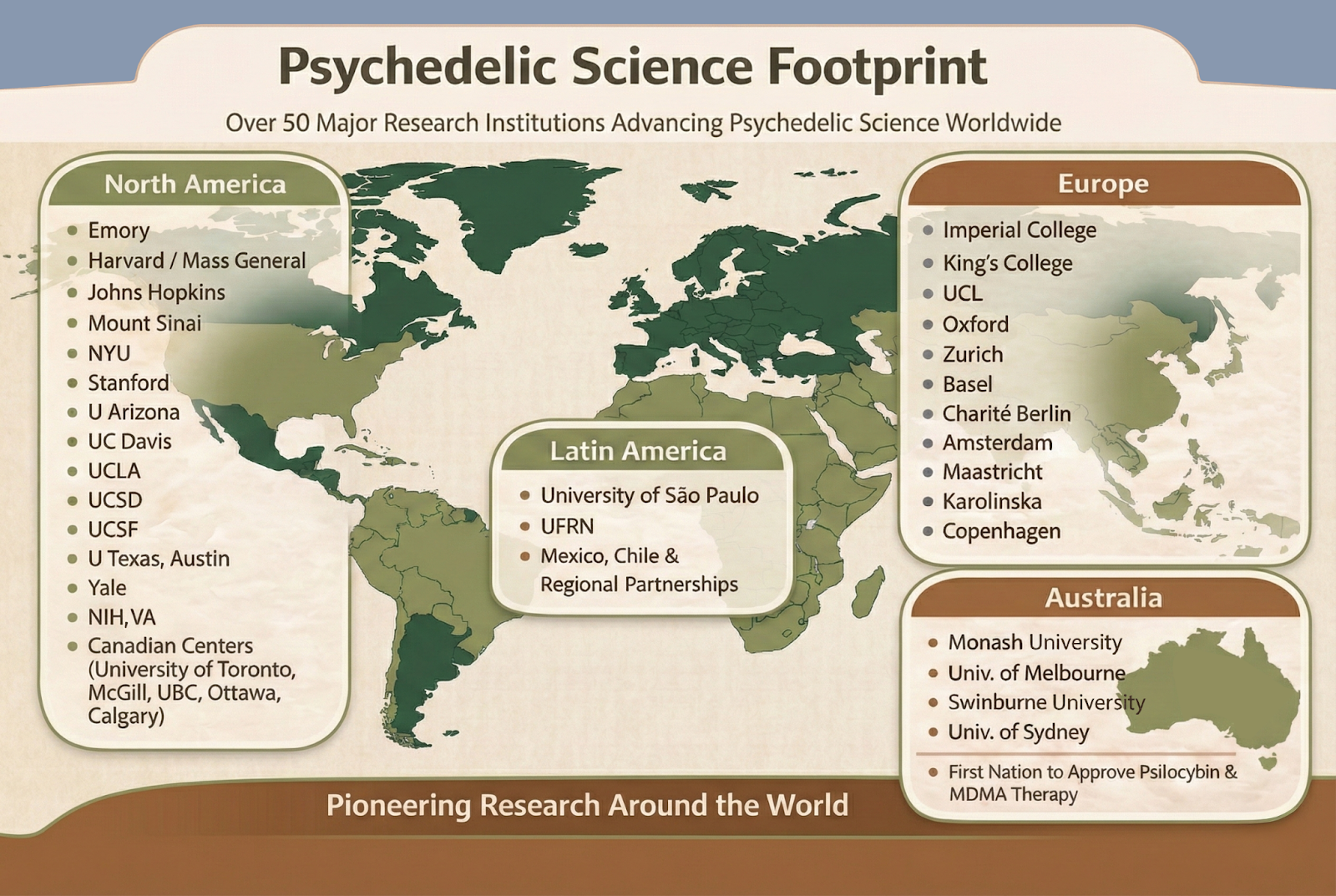
These are not isolated programs. There’s an increasingly coordinated and collaborative global push to truly understand and apply evidence-based psychedelic approaches to mental health and neuroscience conditions.
The Explosion of Peer-Reviewed Research
One of the strongest indicators of mainstream acceptance of psychedelic research is the dramatic growth of peer-reviewed research. Earlier in the 2000s, psychedelic science produced fewer than 20 new publications per year. Today:
- ~300–350 new papers are published annually
- more than 1,500 publications have appeared since 2018
- randomized controlled trials are increasing rapidly
- major journals, conferences, and symposia regularly feature psychedelic neuroscience
Clinical Trials and Regulatory Progress
Psychedelic therapeutic development has moved well beyond exploratory pilot trials. Today, multiple Phase 2 and Phase 3 trials are completed, ongoing, or enrolling, assessing outcomes with multiple compounds including:
- Psilocybin – over 70 human Phase 2 and 3 Clinical trials for indications such as major depression, treatment-resistant depression, anxiety, cancer distress, addiction, OCD.
- MDMA-assisted therapy – two completed Phase 3 PTSD trials demonstrating strong remission with no serious adverse events.
- LSD – advancing through Phase 3 trials for Generalized Anxiety Disorder and Major Depressive Disorder
- DMT & 5-MeO-DMT – structured Phase 2 Trials for Treatment Resistant Depression,
- Ketamine & analogs – over 300 clinical trials defining rapid-acting, neuroplasticity-based treatment paradigms
This is not “alternative” medicine. It is neuroscience-driven innovation following the same rigorous pathway as any modern psychiatric treatment.
What the Evidence Shows
Recent Phase 2 and Phase 3 trials, consistently demonstrate that when psychedelic-assisted therapy with drugs like psilocybin, LSD and MDMA, are delivered in 1-3 supervised dosing sessions by experienced psychedelic practitioners, over weeks or months, a majority of patients experience:
- rapid onset of benefit
- meaningful and sometimes durable outcomes
- very low physiological toxicity
- no addictive potential (except for ketamine with some small risk)
This approach is fundamentally different: a carefully designed procedure for the mind and brain with preparation and integration to support lasting healing, not a daily pill approach. These guided experiences help people reconnect with themselves and their lives.
Why These Therapies May Work: Neuroplasticity
A growing body of human imaging, molecular biology, and animal research suggests that psychedelics act as neuroplastogens, temporarily increasing the brain’s ability to reorganize, adapt, form new connections, and break out of rigid patterns associated with depression, anxiety, addiction, and trauma. Evidence shows:
- increased dendritic spine formation
- strengthened long-range brain connectivity
- reduction in rigid rumination loops
- structural and functional circuit remodeling
- psychotherapy-dependent integration of change
Rather than erasing problems, these therapies may help the brain regain flexibility and responsiveness to therapeutic input.
Where PSI and its TRIP Clinics Fit In
At Psychedelic Science Institute TRIP Clinics in Hollywood and Santa Monica, we are proud to be part of this global movement to better understand brain health and advance responsible psychedelic medicine. Our experienced team is committed to:
- Conducting FDA-regulated clinical trials
- Providing safe, ethical patient care
- Training the next generation of psychedelic practitioners
- Expanding access for underserved communities
We are also committed to being a trusted source of accurate science-based information, helping the public separate hype from reality.
Why This Work Matters
Psychedelic-assisted therapies may represent one of the most promising breakthroughs in mental and brain health in decades. But progress requires resources, funding for research, training, infrastructure, and access programs.
If you believe in the potential of this science to change lives, please consider supporting the nonprofit Psychedelic Innovation Fund (PIF). Your donation directly fuels research, education, and patient care. Together, we can help usher in one of the most transformative eras in mental healthcare in over half a century.
Learn more:
Enrolling Clinical Trials: www.psychedelicsci.com/trials
Ketamine-Assisted Psychotherapy: www.psychedelicsci.com/KAP
PSI Learning Center: www.psychedelicsci.com/learn
Support PIF: www.psychedelicsci.com/pif
The following questions address common public, clinical, and regulatory concerns about psychedelic science and its role in modern medicine.
What is psychedelic-assisted therapy?
Psychedelic-assisted therapy is a structured clinical approach that combines a psychedelic compound, such as psilocybin, MDMA, or ketamine, with professional psychological support. Treatment typically includes preparation sessions, one or more supervised dosing sessions, and integration therapy to help patients process and apply their experiences. Learn more at psychedelicsci.com/learn.
Is psychedelic therapy considered legitimate science today?
Yes. Psychedelic science is now an established area of research within mainstream neuroscience and psychiatry. Leading academic institutions worldwide conduct FDA-regulated clinical trials, and findings are published in peer-reviewed medical journals. Several therapies have received FDA Breakthrough Therapy designation.
What mental health conditions are being studied with psychedelics?
Current clinical research is focused on conditions such as major depressive disorder, treatment-resistant depression, PTSD, anxiety related to serious illness, substance use disorders, and end-of-life psychological distress. Research is also expanding into brain health and neurological conditions, including traumatic brain injury recovery and chronic pain.
Are psychedelic treatments legal in the United States?
Most psychedelic compounds remain federally controlled substances. However, legal access exists through FDA-approved clinical trials, expanded access programs, and, in some states and cities, regulated medical or therapeutic frameworks. Ketamine-assisted therapy is legally available nationwide when provided by licensed clinicians.
How is psychedelic-assisted therapy different from daily psychiatric medications?
Unlike daily medications taken continuously, psychedelic-assisted therapy typically involves a small number of supervised sessions combined with psychotherapy. The goal is not symptom suppression but supporting psychological insight, emotional processing, and longer-term change through enhanced neuroplasticity.
Are psychedelic therapies safe?
Psychedelic-assisted therapies are still being studied and are not yet FDA-approved, which means researchers are continuing to evaluate both their safety and potential benefits.
In carefully controlled clinical research settings, these therapies have generally been well-tolerated when provided under medical supervision, with thorough screening, preparation, and follow-up support. However, they are not appropriate for everyone, and safety depends on factors like a person’s medical history, mental health, and the specific treatment protocol being used.
Participating in a clinical trial means the treatment is still being tested, and part of that process is learning more about who it may help, who it may not be right for, and how to use it as safely as possible.
What does “neuroplasticity” mean in psychedelic research?
Neuroplasticity refers to the brain’s ability to adapt, reorganize, and form new connections. Research suggests certain psychedelics temporarily increase neuroplasticity, which may help patients break rigid thought patterns associated with depression, trauma, and addiction when combined with psychotherapy.
How can someone participate in psychedelic clinical research?
Participation is typically available through FDA-regulated clinical trials conducted at approved research centers. Eligibility depends on specific study criteria, medical history, and diagnosis. Interested individuals can explore active trials through PSI, research institutions or clinical trial registries.
