Inside a Psychedelic Clinical Trial: How They Are Designed & The Participant Experience
.jpeg)
Psychedelic clinical trials are FDA-regulated medical studies that evaluate investigational compounds such as psilocybin, LSD, and DMT for mental health conditions like depression, anxiety, and PTSD. Participants move through structured phases including screening, preparation, supervised dosing, and integration sessions, all conducted in controlled clinical settings with licensed therapists and medical oversight. These trials generate the safety and efficacy data required to determine whether psychedelic medicines can become approved treatments.
What is it like to participate in a psychedelic clinical trial? And how do these studies help turn promising medicines into FDA-approved treatments? Whether you're considering a trial for yourself, supporting a loved one, or simply curious about how modern psychedelic research works, this guide explains the full clinical trial experience, from initial screening to final integration sessions.
Why Psychedelic Clinical Trials Matter
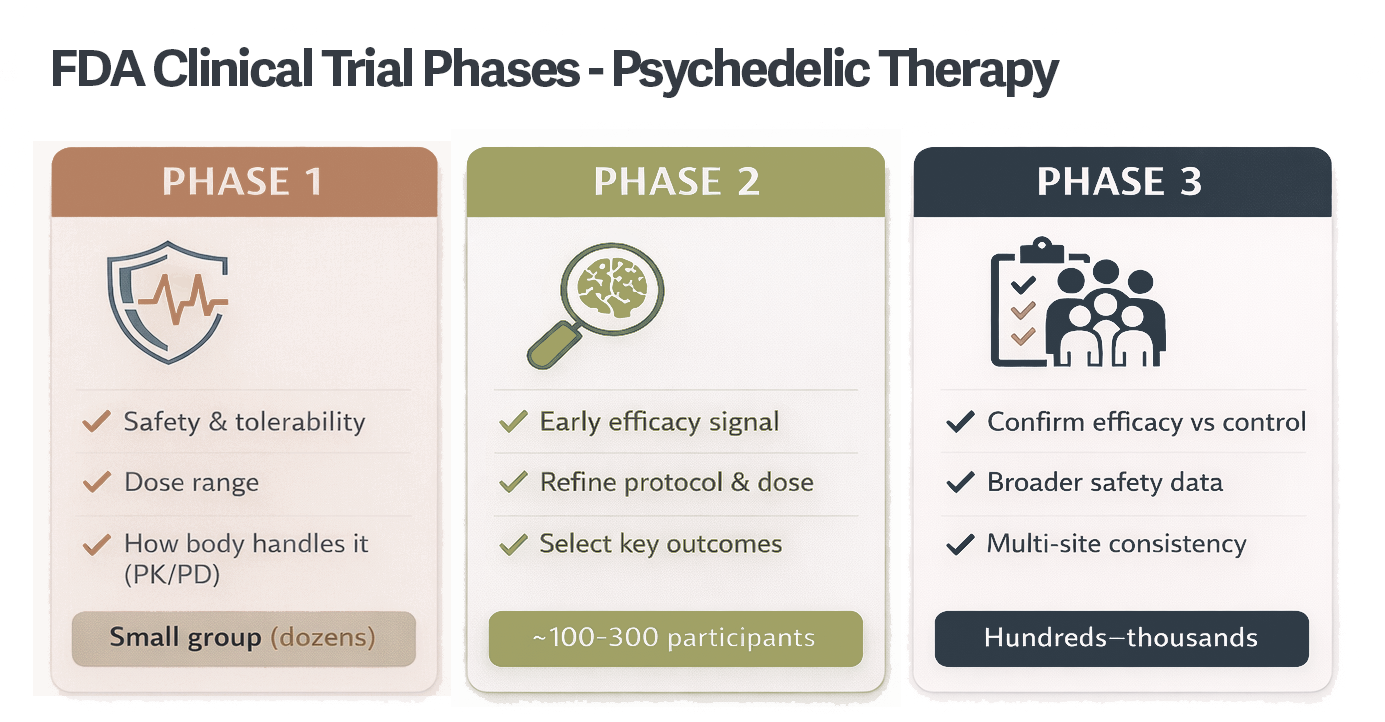
While ketamine-assisted psychotherapy is currently available as an off-label treatment, most psychedelics remain investigational. Clinical trials are the primary pathway for determining whether these drugs are safe, effective, and appropriate for broader clinical use.
Before reaching Phase 2 or Phase 3 trials, these compounds undergo years of early-phase research. By the time participants enroll, researchers are focused on understanding tolerability, symptom changes, durability of effects, and which individuals may benefit most.
Psychedelic research has accelerated significantly in the past decade. Today, more than 30 Phase 2 and Phase 3 randomized, placebo-controlled clinical trials are underway across the United States.
These studies evaluate compounds such as psilocybin, psilocin LSD, DMT, and related investigational medicines for conditions including:
- Major Depressive Disorder (psilocybin, psilocin, LSD)
- Treatment-Resistant Depression (DMT, psilocybin)
- Generalized Anxiety Disorder (LSD)
- PTSD and trauma-related conditions (psilocybin, MDMA)
At organizations like Psychedelic Science Institute (PSI), research is conducted by trained clinicians, therapists, and research staff and each protocol is designed to prioritize participant safety, ethical oversight, and scientific rigor.
Learn more about our ongoing trials at https://www.psychedelicsci.com/clinical-trials.
For deeper educational resources on psychedelic-assisted therapy and research, visit the PSI Learning Center.
Who Can Participate?
Each clinical trial has unique criteria for participation with defined inclusion and exclusion criteria. These criteria protect participant safety and ensure reliable study outcomes.
Eligibility often depends on:
- Confirmed diagnosis and symptom severity
- Age requirements
- Current medications
- Medical and psychiatric history
Some trials require individuals who have never used psychedelics while others allow participants with prior psychedelic experience. All eligibility determinations are made during a structured screening process conducted by the research team.
What Is the Time Commitment?
Most Phase 2 and Phase 3 psychedelic trials last several months to one year. After the initial randomized phase of the study in which some participants receive placebo, most studies include an extension period that may allow eligible participants to receive the active drug.
Typical time commitments include:
- Initial screening visits
- Two or three preparation sessions with licensed therapists
- One or more dosing days, lasting 4 to 12 hours depending on the medicine
- Integration sessions following each dosing experience
- Follow-up visits to monitor safety and symptom changes
Although the commitment is considerable, many participants describe their involvement as meaningful, transformative, and profoundly supportive.
What the Experience Is Like
.png)
Informed Consent and Initial Evaluation:
Your experience begins with a series of conversations and evaluations that help determine whether the study is appropriate for you and if you meet all enrollment criteria. During informed consent, you learn exactly what participation entails, its potential risks, benefits, and unknowns, and the research team encourages you to ask as many questions as you need before deciding whether to enroll.
Medical and Psychological Screening:
The screening process includes interviews, questionnaires, psychological assessments, review of medical records, bloodwork, and an ECG. These steps help the research team understand whether the study is safe and appropriate for you. They also give you time to ask questions and decide whether participation feels right.
Preparation Sessions With Therapists:
Once enrolled, you spend time with your study therapists building rapport, clarifying expectations, and preparing emotionally for the dosing day. These sessions help you develop trust and establish a therapeutic foundation that will support you throughout the trial.
Dosing Day:
The dosing day is typically a full day at the clinic. The dosing room is designed to offer a calm, private, and supportive setting with soft lighting, comfortable furnishings, curated music, and the option to wear eyeshades. Two licensed therapists stay with you throughout the entire session, and medical personnel monitor your physical well-being. Experiences vary by person and through an individual journey or trip. Some participants feel curiosity, insight, or emotional release; others may have periods of discomfort or anxiety. You are supported throughout by the therapists (guides).
Integration Sessions After Dosing:
In the days following the dosing experience, you meet again with your therapy team to explore the emotions, memories, and insights that emerged. Integration helps you make meaning of the experience and translate it into practical, supportive changes in your everyday life. Many participants describe these sessions as one of the most impactful parts of the study.
What If I Receive a Placebo?
Most psychedelic trials are randomized, meaning some participants receive a placebo or a very low dose. To balance scientific rigor with participant access, many studies include an open-label extension.
During this extension, eligible participants may receive the active drug, sometimes multiple times over the course of a year. This phase also helps researchers understand how long benefits may last and whether repeated dosing is necessary.
Potential Risks and Benefits of Participation
Clinical trials are designed to answer research questions, not to guarantee personal benefit. Still, many participants report improvements in mood, anxiety, coping skills, emotional flexibility, and overall sense of connection or meaning.
At the same time, psychedelics can temporarily elicit strong emotions, challenging memories, or perceptual changes. You may also experience nausea, vomiting, headaches, or fatigue. Persistent perceptual changes are exceedingly rare and have not been observed in recent major trials. Continuous monitoring and therapeutic support help maintain safety throughout the process.
Participation also contributes to scientific knowledge. Your involvement helps researchers better understand how psychedelics work, how they influence neuroplasticity, and who is most likely to benefit.
Data Privacy and Participant Confidentiality
Your personal information is protected under strict federal research regulations. Identifying details are removed before your data is analyzed, and only authorized study personnel can access confidential records. Results are examined at the group level rather than individually. Your contribution advances the scientific understanding of psychedelic therapies without compromising your privacy.
Who Oversees These Studies?
Psychedelic clinical trials are highly regulated and are overseen by:
- The FDA, which reviews study design and monitors safety
- The DEA, which regulates handling of Schedule I medicines
- Institutional Review Boards (IRBs), which ensure ethical conduct and participant protection
- Study sponsors, who fund and monitor operations
- Investigators and medical teams, who deliver care and ensure safety throughout the study
These layers of oversight ensure that psychedelic trials adhere to the highest scientific and ethical standards.
What Determines FDA Approval for Psychedelic Medicines?
For any psychedelic medicine to receive FDA approval, it must show consistent, compelling evidence of safety and effectiveness across large, well-controlled studies. It must demonstrate improvement over placebo, show durable benefits, and meet strict manufacturing and quality requirements. At least one successful Phase 3 trial is required before the FDA considers approval.
Is a Psychedelic Clinical Trial Right for You?
People consider enrollment for many reasons: their symptoms are not improving with existing treatments, they are curious about new therapeutic approaches, or they value the structured support and close monitoring that a clinical trial provides. Participation requires commitment and openness to the therapeutic process. If you're unsure whether a trial is the right next step, PSI clinicians can help you explore your options.
How to Get Involved
To learn which trials are enrolling at our PSI TRIP Clinics, visit psychedelicsci.com/clinical-trials. You can complete an interest form to assess eligibility or speak with your clinician about referral options. Family members are also welcome to inquire on behalf of loved ones.
FAQs
What is a psychedelic clinical trial?
A psychedelic clinical trial is a regulated medical study that evaluates the safety and effectiveness of investigational psychedelic compounds under controlled conditions.
What is the dosing day like in a psychedelic trial?
Participants spend several hours in a clinic with two licensed therapists present, while medical staff monitor physical safety throughout the session.
What happens after the psychedelic experience?
Participants attend integration sessions where they discuss insights, emotions, and experiences with their therapy team to support long-term understanding.
Will I definitely receive the psychedelic medicine?
Not always. Most trials include placebo groups, but some offer open-label extensions where eligible participants may later receive the active medicine.

.jpeg)
